Basics of Intraocular Lenses (IOLs)
Intraocular Lenses (IOLs)
For a large portion of human history, the cataractous lens was removed from the eye but without another lens placed, things may have been brighter but with no refracting element things were also blurry. Aphakic spectacles (sometimes called “coke bottle” glasses due to their thickness) certainly was improvement to the aphakia but they were uncomfortable and caused significant distortions as they often had to be around +10 D.
During WWII, a Royal Air Force fighter pilot named Mouse Cleaver was flying a Hawker Hurricane without wearing goggles was shot down and sustained shards of the windscreen in his eye. An observant physician named Dr. Harold Ridley appreciated that this material caused no inflammation and was well tolerated by the eye so he proposed and eventually placed the first acrylic lens in 1949. Without any way to estimate the necessary power, the spherical equivalent refractive error from the first successful IOL was -21.00 D.
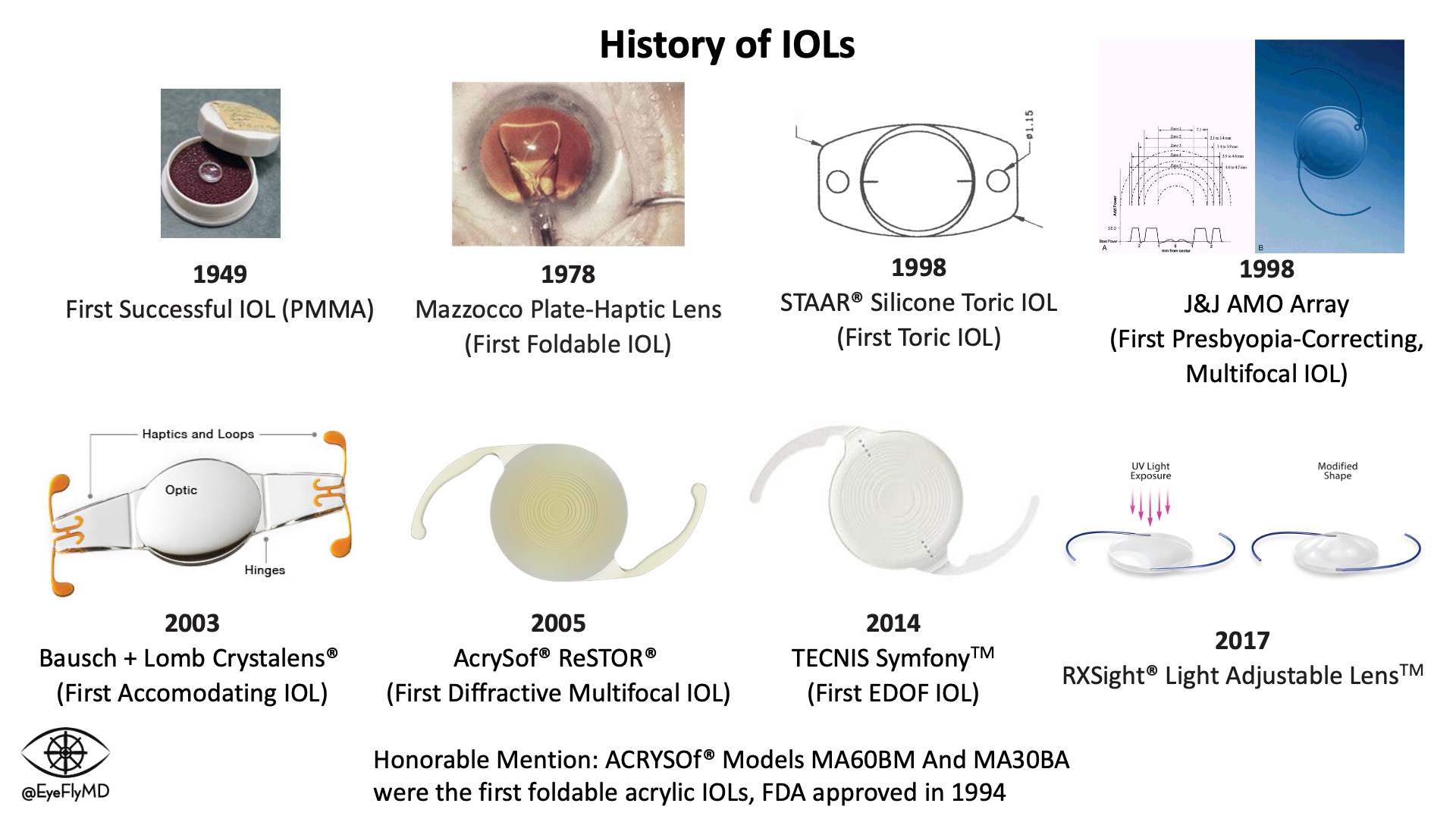
IOLs have undergone an evolution throughout their history as well. Here is a brief summary of their evolution.
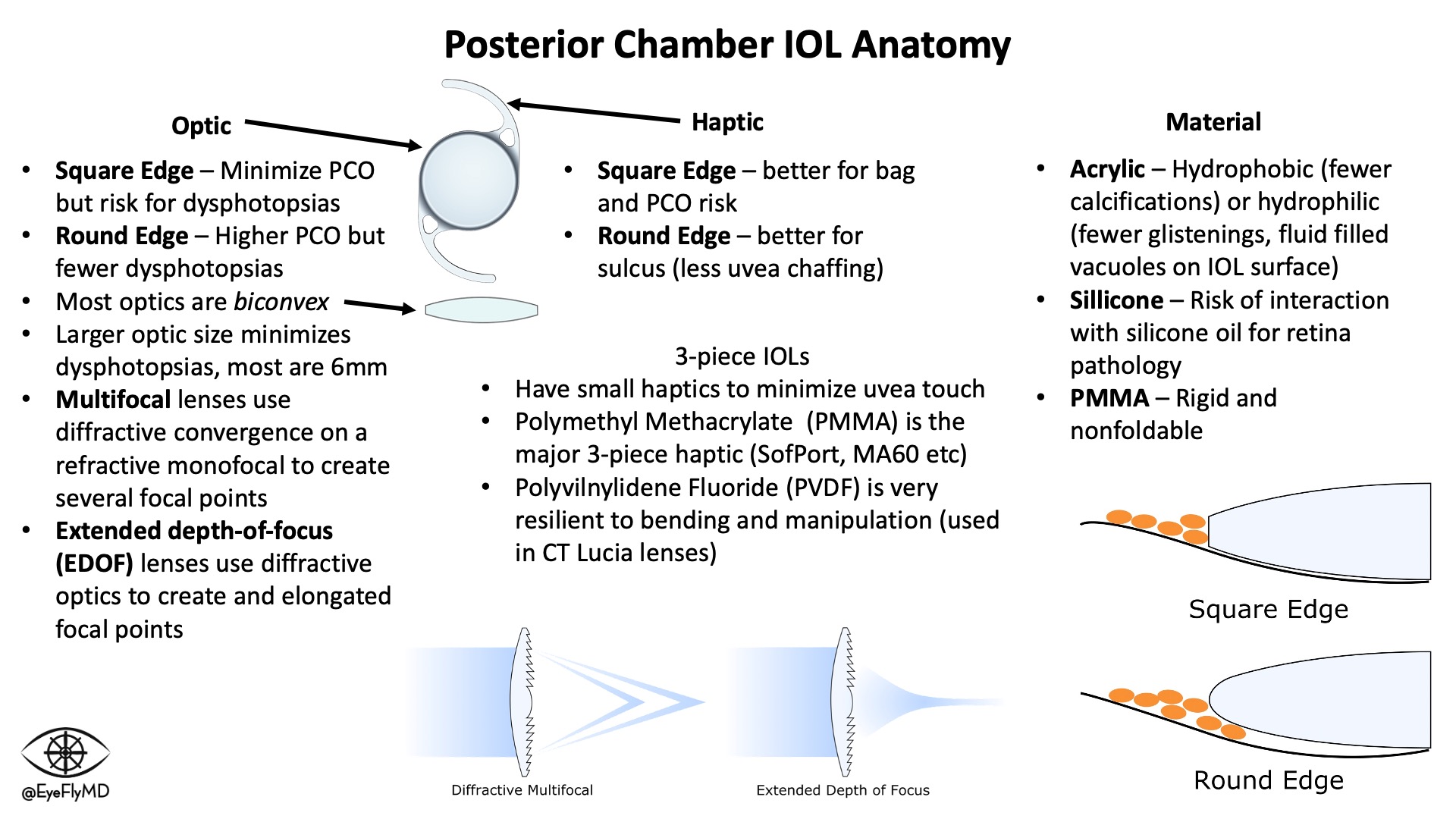
IOLs have a central optic portion and (usually) two haptics that hold the IOL in place in the capsular bag. Keeping the IOL stable in the bag is especially important in toric lenses where rotation would cause reduction in cylinder correction. For every 1° a toric IOL rotates off axis, ~3% of the cylinder power is lost so if the lens rotates 30°, the entire effect of the lens is lost. If it rotates > 30°, the lens starts adding cylinder to the system.
Optics are normally 6 mm. The most common types of IOLs are Acrylic, Hydrophobic (to minimize calcifications), foldable, and square edge (to act as a physical barrier for epithelial cell migration and reduce PCO formation).
3-piece IOLs have small, filament haptics because they are typically placed in the ciliary sulcus so contact with the uveal tissues must be minimized. Most of the time, these haptics are fragile and care must be exercised during manipulation.
There are multiple types of lenses including:
- Monofocal IOL - The standard lens that corrects vision at one distance.
- Toric IOL - A premium option that corrects for corneal astigmatism. A Torus...
- Multifocal IOL - Premium lens (usually ~$2,000 cost to the patient) that corrects vision...
- EDOF IOL - An “Extended Depth of Focus” lens is also a premium lens that uses sophisticated optics to enlarge the distance light is focused on the retina. Patients can still experience halos, glare, and loss of contrast sensitivity.
- Accommodating IOL- Theoretically continues to accommodate like a natural lens.
- Light Adjustable IOL - An IOL that can be “fine-tuned” after surgery using UV light to adjust the final refractive outcome.
Multifocals use a base monofocal and a diffractive element to create an additional focal point. EDOF lenses create an extended focal point by spreading the light. These come at the cost of contrast sensitivity because there is only so much light entering the eye and by using it to spread the light over a larger range or multiple focal points there is inherently a reduction in the amount of photons focusing on each point. Monofocal IOLs still provide the best contrast sensitivity because they focus all available light to a single point (remember no optical system is perfect and some light is always lost).
IOL Anatomy
Comparing IOLs
Modulation Transfer Function (MTF) charts or Defocus Curves (explained below) are a good way to compare lens performances. MTF charts also highlight the concept of “photon allowance” in multifocal and EDOF lenses. The cost of multiple focal points or extended focal ranges is contrast sensitivity as fewer photons form discrete focal points. Surgeons are usually cautious to place premium (multifocal or EDOF) IOLs in eyes with existing or the potential for retinal or other eye pathology (e.g., AMD or glaucoma) because complicating the optical system further will risk suboptimal vision in the setting of disease.
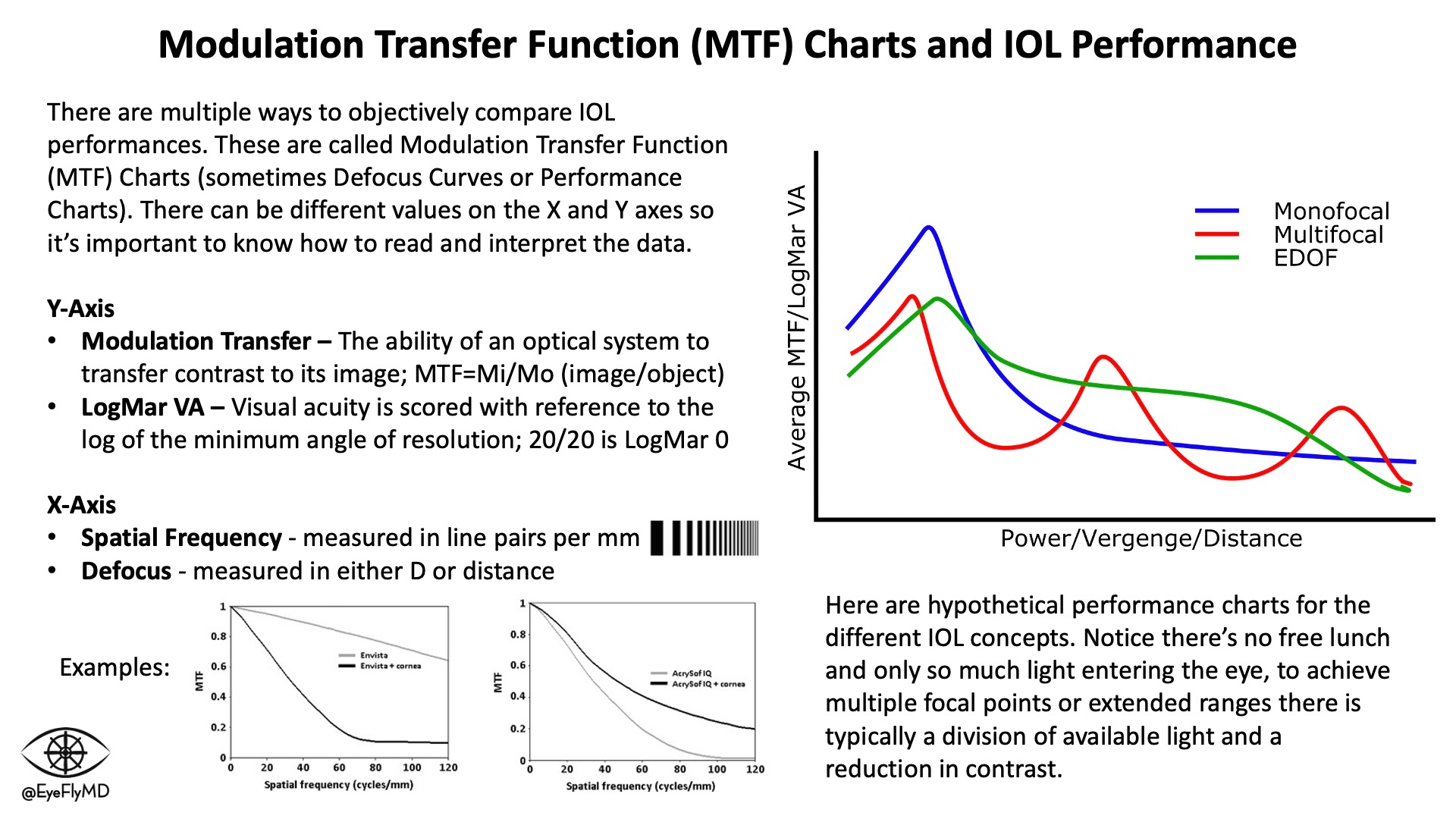
MTF Charts
IOL Calculations
The first major formula was Sanders-Retzlaff-Kraff (SRK). It is now mostly of historical significance but underscores the importance of accurate measurements. Appreciate that a 1 mm mistake in axial length equates to an average of ~2.5 D of error for the lens!
Before cataract surgery, it's important to obtain accurate measurements of the eye (biometry) to accurately calculate the power of lens to use. Lenses are most often placed in the capsule but can also be placed in the ciliary sulcus or anterior chamber. The most important measurements for IOL calculations are axial length and corneal power. Extremes on either end of axial length or corneal power can impact the accuracy of calculations.
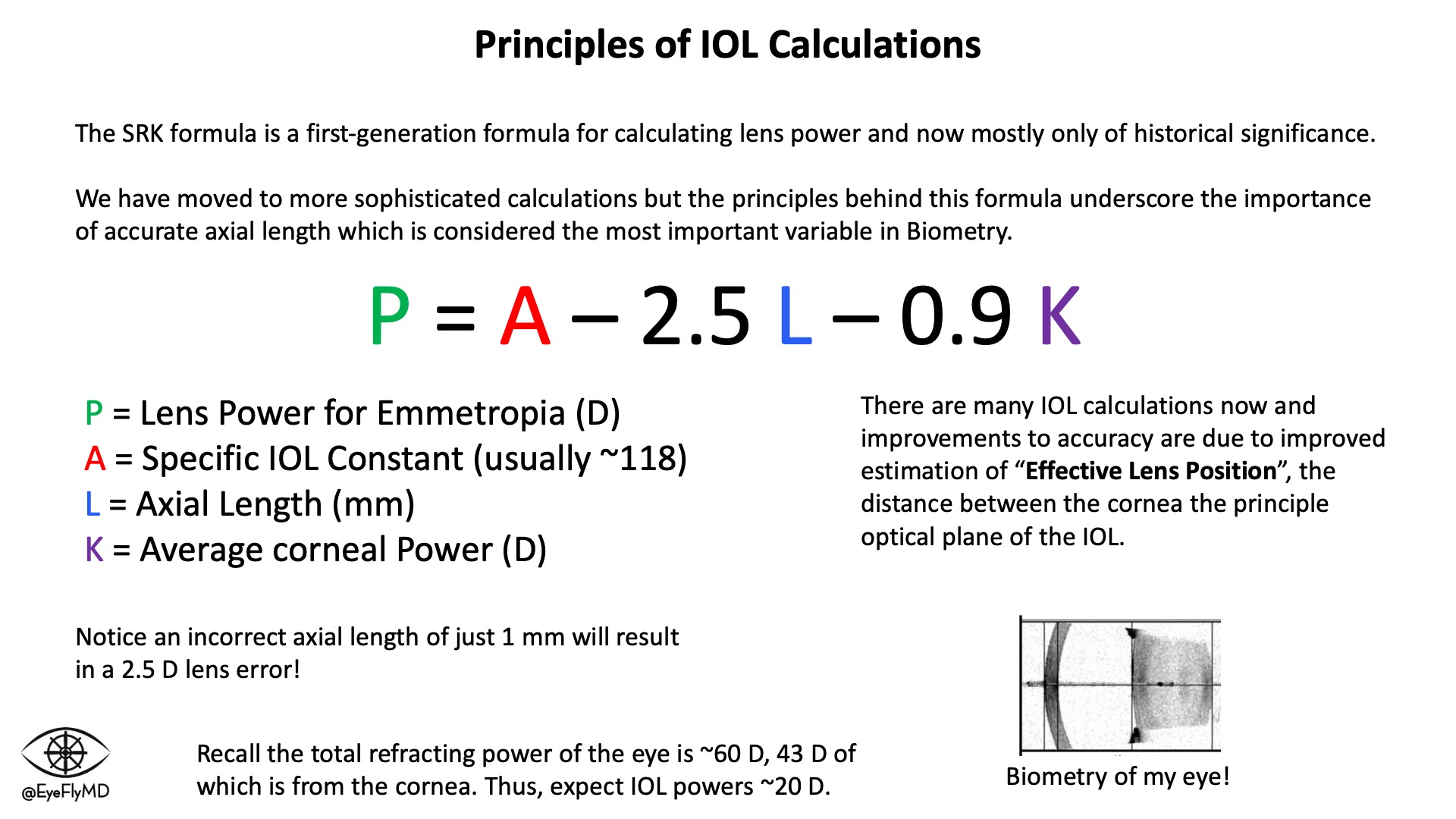
SRK Formula
Just for fun, we can calculate the power needed for an eye. Let's use enVista as an example. It has an A-constant of 119.1. Let’s use L = 24.23 mm and K = 42.25 D. This eye would need:
P = 119.1 - 2.5(24.23) - 0.9(42.25) = +20.50 D
Lens Loading
Loading IOLs is a very common “first step” for students or new residents in the OR. It's crucial to have a strong grasp of how to safely and efficiently load IOLs without damaging them. Some IOLs are preloaded but knowing how to load lenses is crucial. Here are videos that explain how to load single- and 3-piece IOLs.